Argonaute protein is involved in RNAi, it recognizes and binds to siRNA duplex, cleaves and removes passenger strand, and allows guide strand to scan target mRNA.
Argonaute (AGO) proteins have 4 discrete folding units:
- N-terminal: Acts as a wedge.
- PAZ domain: contains a binding pocket, anchors the 2-nucleotide 3′ overhang of siRNA like duplex.
- MID domain: Binds to 5′-P of guide RNA.
- PIWI domain: Adopts a RNase H folds and 3 residues within PIWI domain, form a catalytic triad.
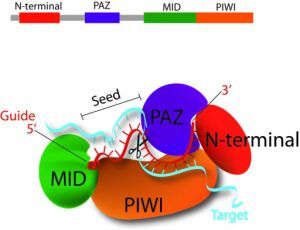
Both ends of the guide RNA remain stably bound to AGO protein. Now the question is how it differentiates guide strand (anti-sense) from passenger strand (sense)?
a. The MID domain consists of a Rossmann-like fold and interacts directly with the 5′-end of the guide strand by recognition of the phosphorylated first nucleotide and base features.
b. the MID domain of RsAgo (bacterial) forms base-specific interactions with the 5′-terminal uracil of the guide RNA strand. The aromatic residue Y463 stacked against the 5′-terminal uracil and the main chain of A454 formed a hydrogen bond with N3 of the uracil. Y463 is widely conserved across Argonaute proteins and this conserved tyrosine has also been shown to stack against the 5′-terminal base of the guide strand in other Argonaute proteins.
It means few features of guide strand are used for recognition by AGO.
Details- Structural basis for the recognition of guide RNA and target DNA heteroduplex by Argonaute

